- India
- Oct 29
Explainer / Pneumococcal Conjugate Vaccine (PCV)
Union Health Minister Mansukh Mandaviya launched the nationwide expansion of the Pneumococcal Conjugate Vaccine (PCV) under the Universal Immunization Programme (UIP).
It is for the first time in the country that the PCV will be available for universal use.
Pneumococcal disease
• Pneumococcal disease is the name given to a group of diseases caused by a bacterium called Streptococcus pneumoniae (also known as pneumococcus).
• The bacteria can cause a number of different types of very serious disease, which can affect the lungs, ears, sinuses and brain. Pneumococcal disease is serious, and can lead to pneumonia, meningitis, bacteraemia/sepsis, sinusitis, bronchitis and middle ear infection.
• Pneumococcal mortality is a significant contributor to the under-5 mortality rate worldwide.
• Pneumococcal pneumonia in particular is a major public health concern for children globally. This infection accounts for 18 per cent of all severe pneumonia cases and 33 per cent of all pneumonia deaths worldwide.
Pneumonia
• Pneumonia is a leading cause of death among children under the age of five globally and in India. Pneumonia caused by pneumococcus is the most common cause of severe pneumonia in children. Pneumonia claims an estimated 8 lakh lives a year globally. More than 80 per cent of deaths associated with pneumonia occur in children during the first two years of life.
• As in the global scenario, pneumonia due to Streptococcus pneumoniae (pneumococcal pneumonia) is responsible for a large portion of pneumonia cases and deaths in India.
• In India, around 16 per cent of deaths in children occur due to pneumonia.
• The nationwide roll out of PCV will reduce child mortality by around 60 per cent.
Types of pneumococcal vaccines
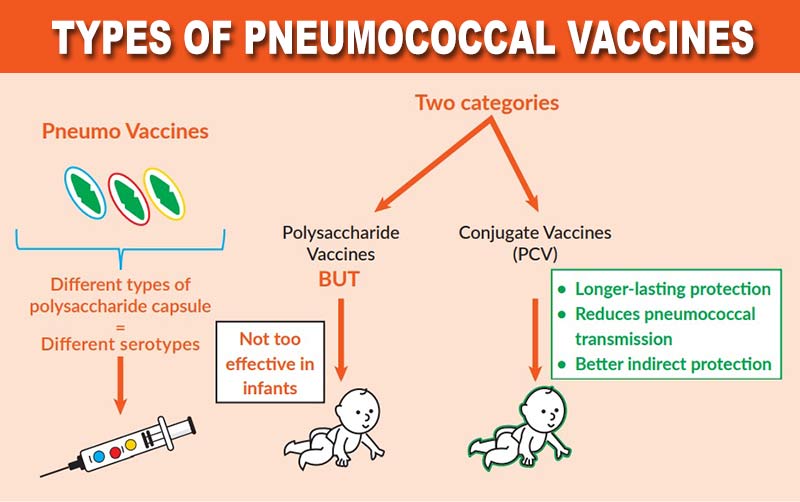
• Pneumococcal vaccines are derived from sugars (polysaccharides) from the capsule of the bacterium Streptococcus pneumoniae. They may or may not be attached to the carrier protein.
Based on the presence of carrier protein, two broad categories of pneumococcal vaccines are available in market:
• Polysaccharide vaccines (with no carrier)
• Conjugate vaccines (with protein carrier).
• Pneumococcal polysaccharide vaccine (PPSV): 23-valent polysaccharide vaccine (PPSV23), available since the early 1980s.
• Pneumococcal conjugate vaccines (PCV): 10-valent (PCV10) and 13-valent (PCV13) are currently available. A 7-valent conjugate vaccine (PCV7), which was introduced in 2000, has been phased out.
• In PCV, each polysaccharide is attached, or conjugated to, a carrier protein. The carrier protein is selected to improve the immune response in those vaccinated.
• In contrast to PCV, PPSV has poor or absent immunogenicity in children under 2 years of age.
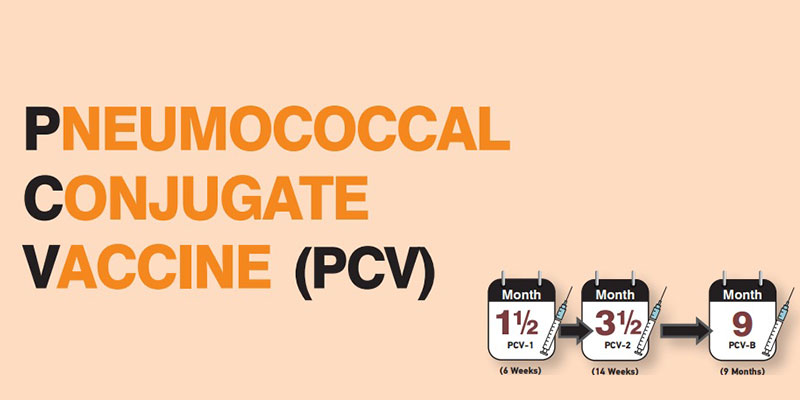
• PCV will be administered in three doses (2 primary and 1 booster) at 6 weeks, 14 weeks and 9 months of age as part of routine immunization.
• PCV has been shown to protect very young children starting at six weeks of age when infants are most at risk of infection. It protects against severe forms of pneumococcal disease, such as pneumonia, meningitis and bacteraemia. It will not protect against these conditions if they are caused by agents other than pneumococcus.
• In a recent report, WHO stated that the currently available PCVs are safe and effective, and the increase in the number of serotypes in these vaccines as compared with the first licensed PCV7 represents significant progress in the fight against pneumococcal disease-related morbidity and mortality, particularly for developing countries.
• India planned for introduction of PCV into its Universal Immunization Programme based on global and Indian evidence and recommendations.
• WHO recommends the inclusion of PCVs in childhood immunization programme worldwide. Use of pneumococcal vaccine should be complimentary to other disease prevention and control measures, such as appropriate case management, promotion of exclusive breastfeeding for the first 6 months of life and reducing known risk factors such as indoor air pollution and tobacco smoke.
Universal Immunisation Programme
• The immunisation programme in India was introduced in 1978 as Expanded Programme of Immunisation (EPI) by the ministry of health and family welfare.
• In 1985, the programme was modified as Universal Immunisation Programme (UIP)
to be implemented in a phased manner to cover all districts in the country by 1989-90 with one of the largest health programmes in the world.
• Universal Immunisation Programme (UIP) is one of the largest public health programmes targeting close to 26.7 million newborns and 29 million pregnant women annually.
• Under UIP, immunisation is being provided free of cost against 12 vaccine preventable diseases.
• Despite being operational for many years, UIP has been able to fully immunise only 65 per cent children in the first year of their life.
• To strengthen and re-energise the programme and achieve full immunisation coverage for all children and pregnant women at a rapid pace, the government launched Mission Indradhanush in December 2014.
• The government identified 201 high-focus districts across 28 states in the country that have the highest number of partially immunised and unimmunised children.
• To further intensify the programme, PM Narendra Modi launched the Intensified Mission Indradhanush (IMI) on October 8, 2017 in selected districts/urban areas with low coverage through targeted interventions focusing on routine immunization micro-planning and greater inter-ministerial/departmental convergence.
Manorama Yearbook app is now available on Google Play Store and iOS App Store