- India
- Nov 28
What are nanozymes?
• Researchers are expanding the horizons of artificial enzymes — nanozymes — to use them as catalysts for transforming biomaterials for their futuristic use in medicinal and biomedical applications.
• Several complex natural enzymes can act on proteins to generate functional proteins. However, the interplay of nanozymes with proteins has rarely been explored.
• Scientists are now probing the unexplored roles of nanozymes in biological environments and their interplay beyond small molecule substrates due to their potential prospects in biotechnological and therapeutic interventions.
• They are also trying to develop next-generation artificial enzymes to overcome the current limitations of selectivity, specificity, and efficiency of existing artificial enzymes.
What are nanozymes?
• Enzymes with intricate structures are powerful biological catalysts designed by nature. Although they perform their action using a high level of sophisticated architecture surrounding their active site, they still have a lot of limitations.
• Their susceptibility to denaturation, the high cost of isolation and purification, short shelf life, and inability to achieve expected catalytic efficiency due to sensitivity to reaction conditions other than their native environment, significantly impede their applications.
• With the advances in research on artificial enzymes, nanomaterial-based enzyme mimetics called nanozymes have attracted significant attention since their inception.
• Nanozymes are a class of nanomaterials with enzyme-like catalytic activities. Due to their multiple catalytic activities, as well as their good stability, modifiable activity and other advantages over natural enzymes, they have a wide range of application prospects in sterilisation, the treatment of inflammation, cancer, and neurological diseases, and other fields.
• In recent years, it has been found that various nanozymes have antioxidant activity, allowing them to simulate the endogenous antioxidant system and play an important role in cell protection.
• Therefore, nanozymes can be applied in the treatment of reactive oxygen species (ROS)-related neurological diseases.
• Another advantage of nanozymes is that they can be customised and modified in a variety of ways to increase their catalytic activity beyond that of classical enzymes.
• In addition, some nanozymes have unique properties, such as the ability to effectively penetrate the blood‒brain barrier (BBB) or to depolymerise or otherwise eliminate misfolded proteins, making them potentially useful therapeutic tools for the treatment of neurological diseases.
• Nanozymes can be divided into the following four categories according to their catalytic activities: oxidoreductases, hydrolases, isomerases, and synthases.
Findings of the study:
• Researchers from the CSIR-Central Leather Research Institute (CLRI), working with the support of INSPIRE Faculty Fellowship and WISE Kiran Fellowship of the Department of Science and Technology (DST), investigated the chemistry at the interface of proteins and nanozymes to push the limits of artificial enzymes.
• They probed the crucial role played by manganese-based oxidase nanozyme (MnN) in stitching collagen, a vital structural protein in various biological tissues, through a covalent process known as “crosslinking” to produce biomaterials.
• In a paper published in Chemical Science, journal of the Royal Society of Chemistry, they showed that MnN can activate collagen with the help of oxidase nanozyme and facilitate the covalent crosslinking of its tyrosine residues using only a trace amount of tannic acid under mild conditions, all the while maintaining the protein's triple-helical structure.
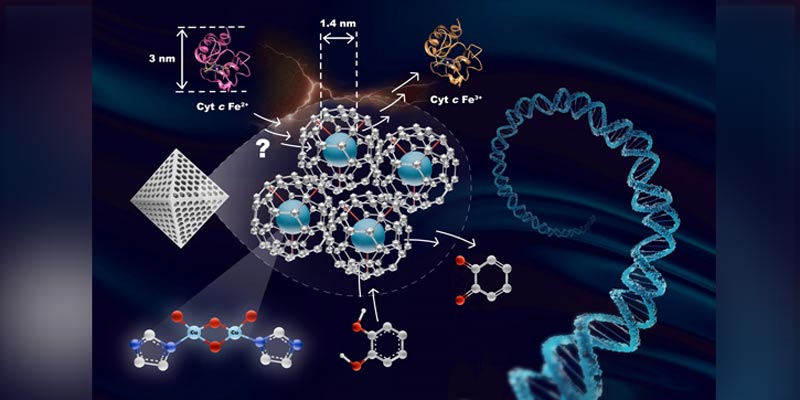
• In another research, the scientists designed a bis-(μ-oxo) di-copper active site installed within the pores of metal-organic framework (MOF-808) to serve as an analogy for enzyme binding pockets and address the persistent challenges of selectivity, specificity, and efficiency in nanozymes.
• Their findings illustrate that while this catalyst-by-design strategy effectively controls substrate dynamics and reactivity, it inadvertently compromises oxidase selectivity when small proteins, such as cytochrome c, which are larger than the pore opening of MOF-808, attempt to access the active site.
• This work exemplifies the need for careful consideration in the meticulous design of artificial enzymes related to nanomaterials, as the refined balance between desirable and undesirable reactivity in artificial enzymes is crucial for medicinal applications.
Manorama Yearbook app is now available on Google Play Store and iOS App Store