- World
- Jul 29
Pfizer, BioNTech pick mNRA vaccine
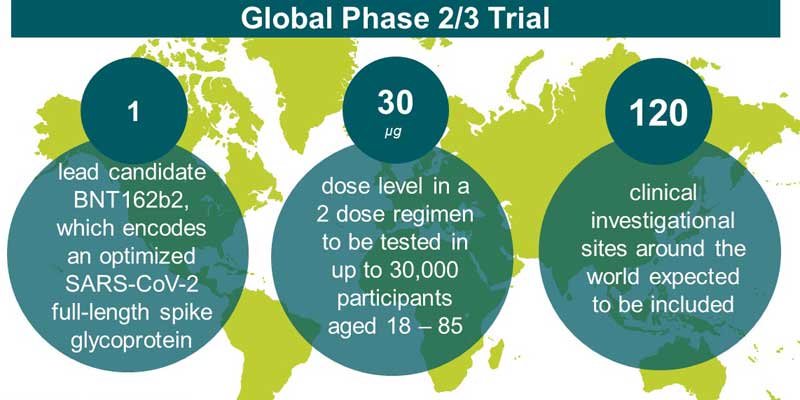
German biotech firm BioNTech and US drugmaker Pfizer Inc announced they would begin a pivotal global study to evaluate their lead COVID-19 vaccine candidate. The study is expected to include about 120 sites globally and could add up to 30,000 participants. It will include regions heavily impacted by COVID-19.
BNT162 mRNA-based vaccine programme
After extensive review of preclinical and clinical data from Phase 1/2 clinical trials, Pfizer and BioNTech have decided to push the single nucleoside-modified messenger RNA (modRNA) candidate from their BNT162 mRNA-based vaccine programme, against SARS-CoV-2 for Phase 2/3 safety and efficacy study.
The vaccine utilises chemical messenger RNA to mimic the surface of the coronavirus and teach the immune system to recognize and neutralise it.
During preclinical and clinical studies of four BNT162 RNA vaccine candidates, BNT162b1 and BNT162b2 emerged as strong candidates based on assessments of safety and immune response. Pfizer and BioNTech selected BNT162b2 as the candidate to progress to a Phase 2/3 study based on the totality of available data from preclinical and clinical studies, including select immune response and tolerability parameters.
In the preclinical studies, BNT162b1 and BNT162b2 candidates induced favorable viral antigen specific CD4+ and CD8+T cell responses, high levels of neutralizing antibody in various animal species, and beneficial protective effects in a primate SARS-CoV-2 challenge model.
Preliminary clinical Phase 1/2 data from nearly 120 patients demonstrated a favorable overall tolerability profile for BNT162b2, as compared to BNT162b1.
Pfizer and BioNTech have chosen to advance their BNT162b2 vaccine candidate into the Phase 2/3 study, at a 30 µg dose level in a 2 dose regimen.
How does mRNA vaccine work?
The premise of any vaccine is to induce a “memory” in the form of B and T cells. Upon any encounter with a pathogen (which can be bacteria or viruses), these cells will recognise the danger and fight it off by destroying the pathogen and pathogen-infected cells.
Messenger RNA (mRNA) is a molecule, composed of nucleotides linked in a unique order to convey genetic information for the cells to produce the proteins or antigens encoded by the mRNA.
Once mRNA in a vaccine is inside of the body’s cells, the cells use their genetic machinery to translate the genetic information and produce the antigens encoded by the mRNA vaccine. The antigens are then displayed on the cell surface, where they are recognised by the immune system which generates a response, including the production of antibodies against the antigen.
Considering the novelty of mRNA vaccines, there isn’t great data clinically when compared to conventional vaccines.
If the study is successful, the companies could submit the vaccine for regulatory approval as early as October, putting them on track to supply up to 100 million doses by the end of 2020 and 1.3 billion by the end of 2021.
Manorama Yearbook app is now available on Google Play Store and iOS App Store