- World
- Jul 25
Various stages of vaccine development
Vaccination is one of the great public health achievements of human history. Immunisation is a global health and development success story, saving millions of lives every year. Vaccines reduce risks of getting a disease by working with your body’s natural defences to build protection. When you get a vaccine, your immune system responds. Vaccines have eradicated smallpox, slashed child mortality rates, and prevented lifelong disabilities.
Immunisation currently prevents 2-3 million deaths every year from diseases like diphtheria, tetanus, pertussis, influenza and measles.
Vaccines are also critical to the prevention and control of infectious-disease outbreaks. They underpin global health security and will be a vital tool in the battle against antimicrobial resistance. Yet despite tremendous progress, far too many people around the world – including nearly 20 million infants each year – have insufficient access to vaccines.
We now have vaccines to prevent more than 20 life-threatening diseases, helping people of all ages live longer, healthier lives.
These diseases include:
Cervical cancer
Cholera
Diphtheria
Hepatitis B
Influenza
Japanese encephalitis
Measles
Meningitis
Mumps
Pertussis
Pneumonia
Polio
Rabies
Rotavirus
Rubella
Tetanus
Typhoid
Varicella
Yellow fever
What is a vaccine?
• The smallpox vaccine, introduced by Edward Jenner — an English physician — in 1796, was the first successful vaccine to be developed in the world.
• A vaccine is a biological preparation that provides active acquired immunity to a particular infectious disease.
• It typically contains an agent that resembles a disease-causing microorganism and is often made from weakened or killed forms of the microbe, its toxins, or one of its surface proteins.
• The agent stimulates the body's immune system to recognize the agent as a threat, destroy it, and to further recognize and destroy any of the microorganisms associated with that agent that it may encounter in the future.
• Vaccines led to the eradication of smallpox, one of the most contagious and deadliest diseases. It also reduced risk from other diseases like rubella, polio, measles, mumps, chickenpox and typhoid.
• Vaccines also help prevent the development of antibiotic resistance.
Stages of vaccine development
Vaccine development is a long, complex process, often lasting 10-15 years and involving a combination of public and private involvement.
1) Exploratory Stage: Work conducted in the basic research laboratory forms the scientific foundation for all subsequent investigation which often lasts 2-4 years. Scientists identify antigens which could include virus-like particles, weakened viruses or bacteria, weakened bacterial toxins, or other substances derived from pathogens. Applied R&D then moves to the clinical research setting, and from there to pilot production and full-scale manufacture.
2) Pre-Clinical Stage: This stage use tissue-culture or cell-culture systems and animal testing to assess the safety of the candidate vaccine and its immunogenicity, or ability to provoke an immune response. Animal subjects may include mice and monkeys. These studies give researchers an idea of the cellular responses they might expect in humans. They may also suggest a safe starting dose for the next phase of research as well as a safe method of administering the vaccine. The preclinical stages often last 1-2 years.
3) Clinical evaluation:
a) Phase I Clinical Trials
Clinical trial is first conducted in a small group of people around 20-80. If the vaccine is intended for children, researchers will first test adults, and then gradually step down the age of the test subjects until they reach their target. Phase I trials may be non-blinded (also known as open-label in that the researchers and perhaps subjects know whether a vaccine or placebo is used). The goals of Phase 1 testing are to assess the safety of the candidate vaccine and to determine the type and extent of immune response that the vaccine provokes.
b) Phase II Clinical Trials
In this stage, the testing population increases to several hundred individuals. Some of the individuals may belong to groups at risk of acquiring the disease. These trials are randomised and well controlled, and include a placebo group. The goals of Phase II testing are to study the candidate vaccine’s safety, immunogenicity, proposed doses, schedule of immunisations, and method of delivery.
c) Phase III Clinical Trials
Phase III trials are much larger in scope involving thousands to tens of thousands of people. They are randomised and double blind and involve the experimental vaccine being tested against a placebo (the placebo may be a saline solution, a vaccine for another disease, or some other substance). To understand the vaccine safety and certain discrepancies that might pop up in the smaller groups of subjects tested in earlier phases, are the major objective of this phase. After successful completion, and once the proper approval and license is in place, market production can begin.
d) Phase IV
Such testing occurs after the license and other approvals for production are in place. This is to ascertain the quality of the vaccine since some rare side effects may not have been detected in the Phase III trials, and thus the vaccine safety is continually monitored by the nodal agencies.
Types of vaccines
Vaccines contain dead or inactivated organisms or purified products derived from them. There are several types of vaccines.
1) Inactivated: These vaccines use the killed version of the germ that causes a disease. Inactivated vaccines usually don’t provide immunity (protection) that’s as strong as live vaccines and hence several doses over time (booster shots) in order to get ongoing immunity against diseases.
Example: Hepatitis, Flu, Polio, Rabies
2) Live-attenuated: Live vaccines use a weakened (or attenuated) form of the germ that causes a disease. Because these vaccines are so similar to the natural infection that they help prevent, they create a strong and long-lasting immune response. Just one or two doses of most live vaccines can give a lifetime of protection against a germ and the disease it causes. However, there are some limitations. Since they contain a small amount of the weakened live virus, it's not advisable for people with weakened immune systems, long-term health problems, or people who’ve had an organ transplant.
Example: MMR Vaccine (Measles, Mumps, Rubella), Rotavirus, Smallpox, Chickenpox, Yellow fever.
3) Toxoid: These vaccines use a toxin (harmful product) made by the germ that causes a disease. They create immunity to the parts of the germ that cause a disease instead of the germ itself. That means the immune response is targeted to the toxin instead of the whole germ.
Example: Diphtheria, Tetanus
4) Subunit: This vaccine uses a fragment of inactivated or attenuated microorganism to create an immune response.
Example: Hepatitis B virus that is composed of only the surface proteins of the virus and vaccine against human papillomavirus (HPV) that is composed of the viral major capsid protein.
5) Conjugate: Such vaccines have polysaccharide outer coats of the micro-organism which led to be recognized as an antigen and triggers immune response.
Example: Haemophilus influenzae type B vaccine
6) Heterotypic: Also called Jennerian vaccines as it contains pathogens of other animals that either do not cause disease or cause mild disease in the organism being treated.
Example: BCG
7) Experimental vaccines: Include Dendritic cell vaccines (contains Dendritic cells with antigens), DNA vaccines (contains infectious agent’s DNA), Recombinant vaccines (DNA encoding antigen to bacterial cells and then extracting the expressed antigen), RNA vaccines (contains infectious agent's RNA within a vector), Peptide vaccines, etc.
Race for COVID-19 vaccine
Severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) was first reported in Wuhan in China in December 2019.
As on July 25, more than 15 million people have been infected by the novel coronavirus and the death toll crossed the six-lakh mark. The pandemic devastated economies across the globe and millions lost their jobs.
A vaccine is urgently needed to prevent COVID-19 and thereby stem complications and deaths resulting from transmission of the disease.
Governments, philanthropies, international organisations, scientists and manufacturers have undertaken efforts to expedite research and development (R&D) for COVID-19 vaccine, as well as other medical products like diagnostic tests.
According to reports, there are more than 150 vaccine candidates to combat the coronavirus in the different stages of trial across the world. More than 20 vaccines are in clinical evaluation.
According to a report, the global COVID-19 vaccines market is projected to reach $2,273 million in 2022 and reduce to $1,401 million by 2025. The growth of the global COVID-19 vaccines market is majorly attributed to the increasing number of people infected with COVID-19 and growing funding for vaccine development.
The global COVID-19 drugs market is projected to reach $165 million in 2020 and reduce to $2 million by 2025. The growth of COVID-19 drugs market is attributed primarily to use of repurposed drugs for compassionate use and the emergence of alternative therapies such as convalescent plasma therapy which were used earlier for treating epidemic diseases such as SARS, MERS, and H1N1.
Challenges of vaccine development
1) Pandemic uncertainty: Market plays a vital role in deciding the potential of vaccines. Since the vaccine takes several years for its full scale production, the incidence of disease declines and thus rapid trial becomes difficult leading to shelving the vaccine in the middle causing huge loss to the government, private agencies. Example: Ebola vaccine
2) Working solution: Given the need for rapid development, many of the regulatory hurdles have to be relaxed to speed up processes for developing the vaccine.
3) Mass production: To meet the production demand at such a massive scale, it's important that the industry starts in parallel with the research – long before clinical results become available. Hence various stakeholders such as scientists, regulatory bodies and industry need to work together.
4) Last mile reach: Distribution is going to be another challenge, particularly when it comes to the third world countries where the incidences are more and the government is not able to afford the cost at such a massive scale. A vaccine would need to reach all corners of the world in order to effectively tackle the virus.
5) Rich countries need to rescue the third world population: Many governments of low-income countries may not be able to pay the costs of deploying a new COVID-19 vaccine.
6) First line of defence to be strengthened: Impact of COVID-19 on frontline health workers is distressing, especially in low- to middle-income countries, where the depletion of health care workers’ ranks could also weaken existing programmes to vaccinate people against other diseases.
7) Formal identification of population: Most people in poor countries lack a formal identity and many are on the move. It will be difficult to keep a track of vaccines and the dose received by this critical mass of dispersed people. Corruption, leakage, and even accidental duplication waste precious supply and are deadly.
8) Data to understand the pattern: It's important to understand the risk of transmission at the hyperlocal level and the pattern of adherence in specific geographies and sub-populations.
9) Logistics & supply chains: Most vaccines need to be kept between 2-8 degrees Celsius. However, in many low- and middle-income countries, electricity sources are unreliable and so stock maintenance of vaccines becomes challenging.
Vaccine development in India
There are two Indian vaccine candidates that have undergone successful toxicity studies in rats, mice and rabbits and this data was submitted to the DCGI, following which both got clearance to start an early phase human trial.
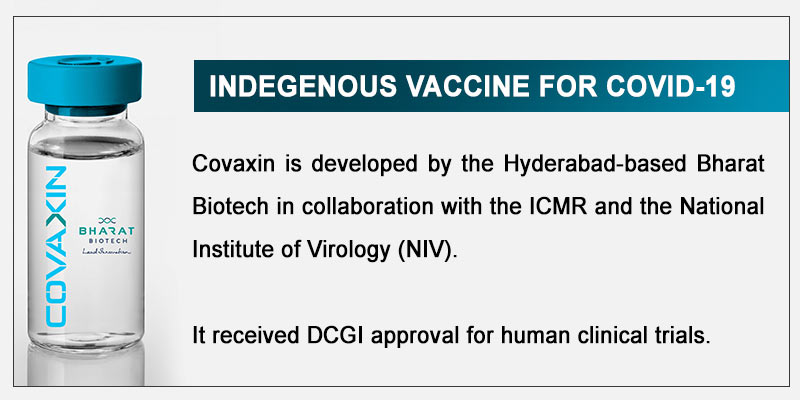
1) Covaxin
Covaxin has started a randomised, double-blind, placebo-controlled clinical trial in 375 volunteers with good results so far.
The ‘inactivated’ vaccine has been developed by the Hyderabad-based pharmaceutical company Bharat Biotech in collaboration with National Institute of Virology (NIV) and Indian Council of Medical Research (ICMR).
The first phase of the trials for Covaxin are expected to take over a month to complete. Then the data will be submitted to the Drug Controller General of India. Following this, it will move on to phase II trials.
2) ZyCoV-D
Another potential COVID-19 vaccine indigenously developed by the Ahmedabad-based Zydus Cadila Healthcare Ltd has got nod from the DCGI for human clinical trials. This plasmid DNA vaccine is named ZyCoV-D.
Its phase I and II trials target 1,048 participants and are to be conducted at one site–Zydus Research Centre in Ahmedabad. ZyCov-D’s first phase of trials is expected to take nearly three months to complete, following which the vaccine will move on to the second phase.
Manorama Yearbook app is now available on Google Play Store and iOS App Store